Finally there are three atoms of oxygen. A sample of calcium carbonate C a C O 3 has the following percentage composition.

Question Video Calculating The Mass Of Calcium Carbonate Required To Produce A Given Mass Of Calcium Oxide Nagwa
Ca 1 40 40.

. A low carbon dioxide and warm temperatures b lots of carbon dioxide and cold temperatures. It is composed of three elements namely Calcium Carbon and Oxygen. We review their content and use your feedback to keep the quality high.
Low pressure and warmer temperatures e. Which of the following contains calcium carbonate CaCO3. Calcium Carbonate is the carbonic salt of calcium CaCO3.
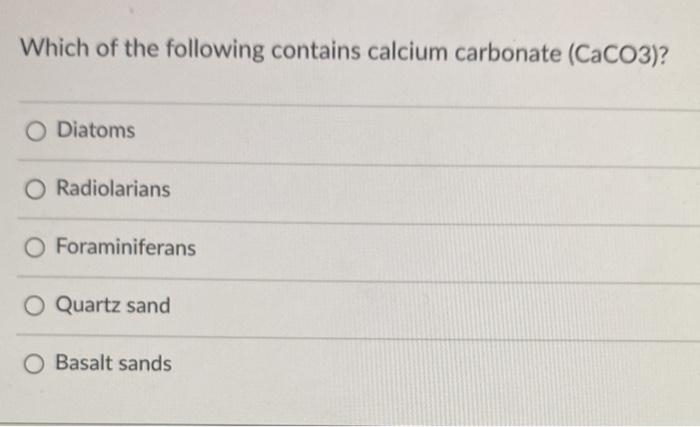
Which of the following contains calcium carbonate CaCO3. A diatoms b foraminiferans c glauconite d phosphorites e radiolarians. It is a solid compound which is white non-toxic and odorless.
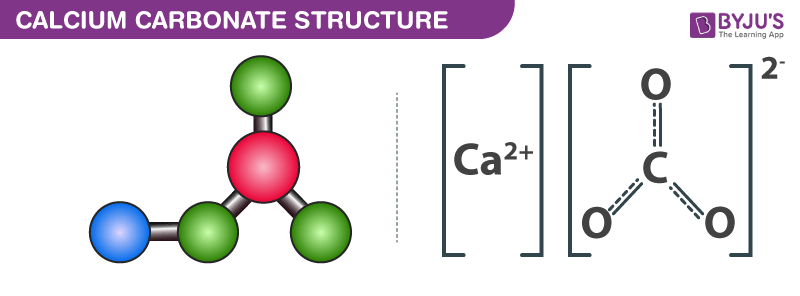
Which of the following contains calcium carbonate CaCO3. The correct answer is C CaCO3 heres why. The formula for calcium carbonate is CaCO3 so the elements involved are calcium Ca carbon C and oxygen O.
C a 4 0. CaCO3 s2HCl aq-CaCl2 aqH2O lCO2 g Tums an antacid contains CaCO3. Calcium carbonate is most likely to dissolve in water with which characteristics.
Medicinally it is used as an antacid or as a calcium supplement. If Tums is added to 350 mL of 0300 M. Low pressure and colder temperatures.
Add all the masses together to. This problem has been solved. If the law of constant proportions is true then the weight of calcium in 4 g of a sample of calcium carbonate from another source will be.
CaCO3 2Hg Caaq CO2 8 H2O note 1 mol CaCO3 neutralizes 2 mol H A Tums tablet containing 1000. C 1 C 3. See the answer See the answer See the answer done loading.
Foraminifera are very small marine organisms which to protect themselves produce shells of calcium carbonate CaCO3. Lots of carbon dioxide and warmer temperatures d. It is also used as fillers in cosmetics.
Mass of Carbon C 12. The mass of Ca is 40078 g. So one molecule has only one atom of calcium which is.
Mass of Calcium Ca 40. Which of the following contains silica SiO2. See answer 1 Best Answer.
Mass of Oxygen O 16. O 4 8. O 3 16 48.
C 1 12 12. Multiply each element mass by the number of atoms of that element. What is the expected calcium carbonate content in modern surface sediments at a.
From the question we were told that Calcium carbonate contains the following. Then there are one atom of carbon. Ca 1 Ca 2.
The chemical formula of calcium carbonate is CaCO 3. Who are the experts. Ca 1atom.
Calcined gypsum is calcium sulphate hemihydrate CaSO 4. Dolomite is an anhydrous carbonate mineral composed of calcium magnesium carbonate ideally CaMgCO 3. O Diatoms O Radiolarians Foraminiferans O Quartz sand O Basalt sands.
It is a white insoluble powder-like substance which occurs naturally in minerals chalk marble limestone calcite shells pearl etc. C 1 2. Is 47997 g 15999 g 3.
O 3atoms. Experts are tested by Chegg as specialists in their subject area. Low carbon dioxide and warmer temperatures b.
Combining the above together we will have. Lots of carbon dioxide and colder temperatures c. It is a chemical compound with the chemical formula CaCO3.
The compound CaCO3 contains the following elements. The molecular weight of the calcium carbonate can be calculated as follows. Sea hells contain a chemical compound called calcium carbonate CaCO 3.
Calcium carbonate CaCO3 reacts with stomach acid HCl hydrochloric acid according to the following equation. See answer 1 Best Answer. Tums a common antacid contains calcium carbonate CaCO3 which neutralizes acid by the reaction.
Calcium Ca Carbon C Oxygen O 1 Calcium Atom 1. Which of the following contains calcium carbonate CaCO3. Which of the following contains calcium carbonate CaCO3.
You take the atomic mass of calcium over the atomic mass of CaCO3 and multiply it by 100 to get the percentage. O O3 Good luck with your studies I hope this helps. Calcium carbonate is most likely to dissolve in water with which characteristics.
The mass of C is 1201 g and the mass of 3O. Calcium Ca CarbonC oxygen O2 Wiki User. Calcium carbonate is used therapeutically as a phosphate buffer in hemodialysis as an antacid in gastric hyperacidity for temporary relief of indigestion and heartburn and as a calcium supplement for preventing and treating osteoporosis.
CaCO3 is a chemical formula for the compound called Calcium Carbonate. Practically the earths own crust contains this mineral making it one of the most abundant raw materials that can be sourced from nature.
Solved Which Of The Following Contains Calcium Carbonate Chegg Com
Limestone Calcium Carbonate Caco3 Uses Preparation Properties Formula Structure Of Calcium Carbonate
0 Comments